Definition of Polymerization
what does polymer mean?
Polymer means many monomers. Sometimes polymers are also known as macromolecules or large-sized molecules. Usually, polymers are organic but not necessarily
we can what is monomer and polymer?
monomer
Monomers are molecules typically about 4-10 atoms in size, reactive in that they bond readily to other monomers in a process called polymerization. Polymers and their polymerization processes are so diverse that a variety of different systems exist to classify them. One major type of polymerization is condensation polymerization, where reacting molecules release water as a byproduct. This is the means by which all proteins are formed.
The monomer molecules may be all alike, or they may represent two, three, or more different compounds. Usually at least 100 monomer molecules must be combined to make a product that has certain unique physical properties—such as elasticity, high tensile strength, or the ability to form fibres—that differentiate polymers from substances composed of smaller and simpler molecules; often, many thousands of monomer units are incorporated in a single molecule of a polymer (Encyclopedia of Britannica)
Polymer definition.
Polymers are molecules which consist of a long, repeating chain of smaller units called monomers. Polymers have the highest molecular weight among any molecules, and may consist of billions of atoms. Human DNA is a polymer with over 20 billion constituent atoms. Proteins, or the polymers of amino acids, and many other molecules that make up life are polymers. Polymers are the largest and most diverse class of known molecules. They even include plastics.(wise geek.com)
a chemical reaction in which two or more molecules combine to form larger molecules that contain repeating structural unit.
polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains (wikipedia.com)
How Polymer formed?
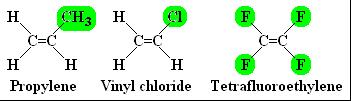
To get a clear idea of the way polymers are formed, you need to look more closely at the monomer molecules! There are many monomer molecules. Here are some examples from materialworld.org,
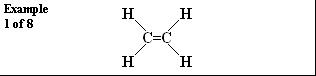
Chain-growth polymerization or addition polymerization involves the linking together of molecules incorporating double or triple chemical bonds. These unsaturated monomers (the identical molecules which make up the polymers) have extra internal bonds which are able to break and link up with other monomers to form the repeating chain.
The highlighted areas show the side groups on these monomer molecules. These groups give the polymer chain some of its properties.
The double bond, however, is the vital feature that allows these monomers to form the long polymer chains.
Chemical Bond
to learn more about polymerization, we must know about chemical bonding concept
A chemical bond is the physical process responsible for the attractive interactions between atoms and molecules, and that which confers stability to diatomic and polyatomic chemical compounds. The explanation of the attractive forces is a complex area that is described by the laws of quantum electrodynamics. In practice, however, chemists usually rely on quantum theory or qualitative descriptions that are less rigorous but more easily explained to describe chemical bonding. In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. Molecules, crystals, and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure of matter.
Examples of Lewis dot-style chemical bonds between carbon C, hydrogen H, and oxygen O. Lewis dot depictures represent an early attempt to describe chemical bonding and are still widely used today. (wikipedia.org)


